Common principles of protein biosynthesis
A current theory states that it was RNA that stood at the beginning of life on our planet (Gesteland, R.F., Cech, T.R., 1999). Yet, the later appearing proteins outperformed RNA in so many fields that those early, self-sufficient RNA constructs are now extinct. Whereas RNA is composed by a combination of basically four different types of nucleotides and is relatively limited in its ability to form tertiary structure, proteins are highly flexible chains built from twenty amino acids that can fold in a larger variety of three-dimensional shapes. The amount of building blocks (twenty compared to only four in RNA – leaving aside base modifications) equips them with a high degree of adaptability to different tasks and might be the reason for their superiority to RNA in many fields ( Figure 1 ).
To build such a peptide chain, amino acids have to be linked in the correct order via peptide bond formation. Although there exist proteins which are able to catalyze peptide bonds, like the sortase (Mazmanian et al., 1999), in all kingdoms of life the responsibility for building these peptide chains lies in a ribonucleoprotein particle: a macromolecular machine called the ribosome.
General features of the ribosome
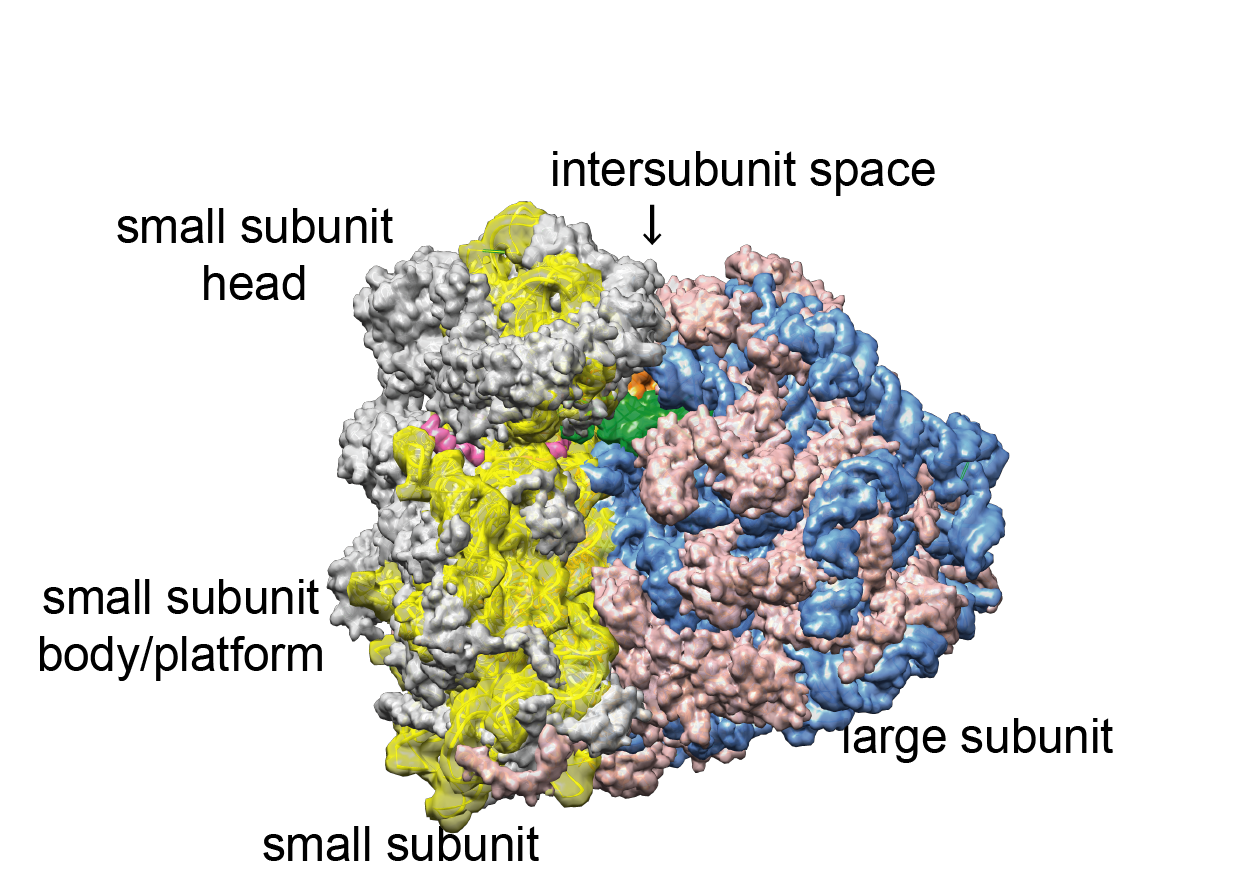
The ribosome consists of a large and a small subunit. Both are made up of ribosomal RNA (rRNA) and ribosomal proteins ( Figure 1 ). The basic mechanism of protein synthesis is very similar in all domains of life: A messenger RNA (mRNA) contains the sequence of the protein and is bound and read by the small ribosomal subunit in collaboration with specific transfer RNAs (tRNAs). tRNAs carry the amino acids and contain characteristic anticodons, which establish base pairing with the respective codons on the mRNA presented to them by the ribosome. Matching allows for addition of the amino acid to the growing peptide chain. After all amino acids have been added, the peptide chain is released from the ribosome and, if needed, further processed by other cell components to become the final, folded protein.
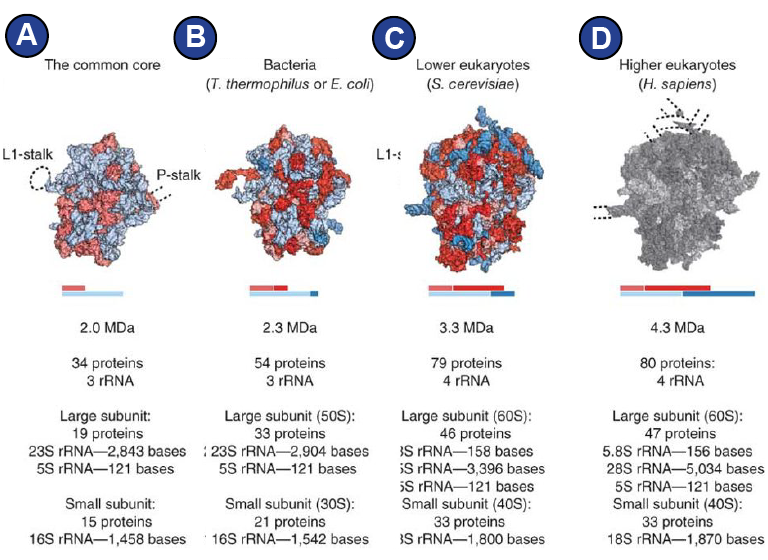
Although the three domains of life, bacteria, eukaryotes, and archaea, all possess ribosomes for protein synthesis, the composition of the ribosomes varies, leading to sometimes very different overall appearance of ribosomes from different kingdoms of life (Amunts et al., 2015; Behrmann et al., 2015; Dunkle et al., 2011; Melnikov et al., 2012; Ramrath et al., 2018) ( Figure 2 ).
The eukaryotic cytoplasmic 80S ribosome is larger than the bacterial 70S ribosome, containing additional RNA segments and additional proteins. Comparison reveals that the eukaryotic ribosome possesses the same conserved structures as the bacterial ribosome in its core (Figure 2A) and an outer shell where eukaryote-specific elements are located ( Figure 1 , Figure 2 ) (Melnikov et al., 2012).
Not only the ribosomes themselves differ by certain features and are differently sensitive to antibiotics (Yusupova and Yusupov, 2017), but also the interacting factors that render translation possible are for some stages of translation remarkably different (Andersen et al., 2006) and might be an expression of the way the ribosome has been optimized for its bacterial, archaean, or eukaryotic cell environment.